Trethera Receives $1.8 Million NIH Grant to Advance TRE-515 Development for Crohn’s Disease
LOS ANGELES, Sept. 23, 2025 (GLOBE NEWSWIRE) -- Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company developing first-in-class therapies for cancer and autoimmune diseases, announced today that the National Institutes of Health (NIH) awarded Trethera a $1.8 million grant for preclinical studies of TRE-515 as a Crohn’s disease treatment. Trethera’s lead drug candidate, TRE-515, is a once-daily oral therapy that inhibits deoxycytidine kinase (dCK), an enzyme critical to the deoxyribonucleoside salvage pathway driving abnormal cell proliferation in autoimmune diseases such as Crohn’s.
This award follows Trethera’s prior $400,000 NIH Crohn’s research grant and builds on the encouraging findings presented at the 2025 Crohn’s & Colitis Congress. The presentation highlighted compelling preclinical data demonstrating that TRE-515 outperformed Johnson & Johnson’s market-leading drug, Stelara. TRE-515 blocked inflammatory bowel disease symptoms in mice by selectively limiting activated CD4 T-cell proliferation.
“This continued NIH funding validates Trethera’s innovative approach of targeting dCK, a key metabolic vulnerability in autoreactive immune cells causing Crohn’s disease,” said Dr. Ken Schultz, Chairman & CEO of Trethera. “We are accelerating TRE-515 development as a first-in-class therapy for Crohn’s patients with high unmet medical needs.”
Crohn’s disease is a chronic, relapsing inflammatory disorder of the gastrointestinal tract that can cause diarrhea, fatigue, severe abdominal pain, and weight loss. Affecting more than 1 million Americans, it remains a debilitating condition marked by recurring flares, persistent intestinal inflammation, and complications such as colorectal cancer. Up to one-third of patients fail to respond to first-line approved therapies, and nearly half lose response within five years.
In its written summary, the NIH peer review panel commented, “TRE-515 is the only dCK inhibitor in development, so this approach is highly innovative… supported by strong preliminary data that TRE-515 is non-inferior to current standard of care therapies… TRE-515 is not strongly immunosuppressive and has a favorable safety profile… oral administration will make it especially attractive… exciting potential and the commercialization plan is exceptional.”
Beyond Crohn’s disease, TRE-515 is also being evaluated in Phase 1 clinical trials for solid tumors and ALS (Lou Gehrig’s disease). With dual potential across cancer and inflammatory diseases, the TRE-515 development program represents a broad platform to transform patient outcomes.
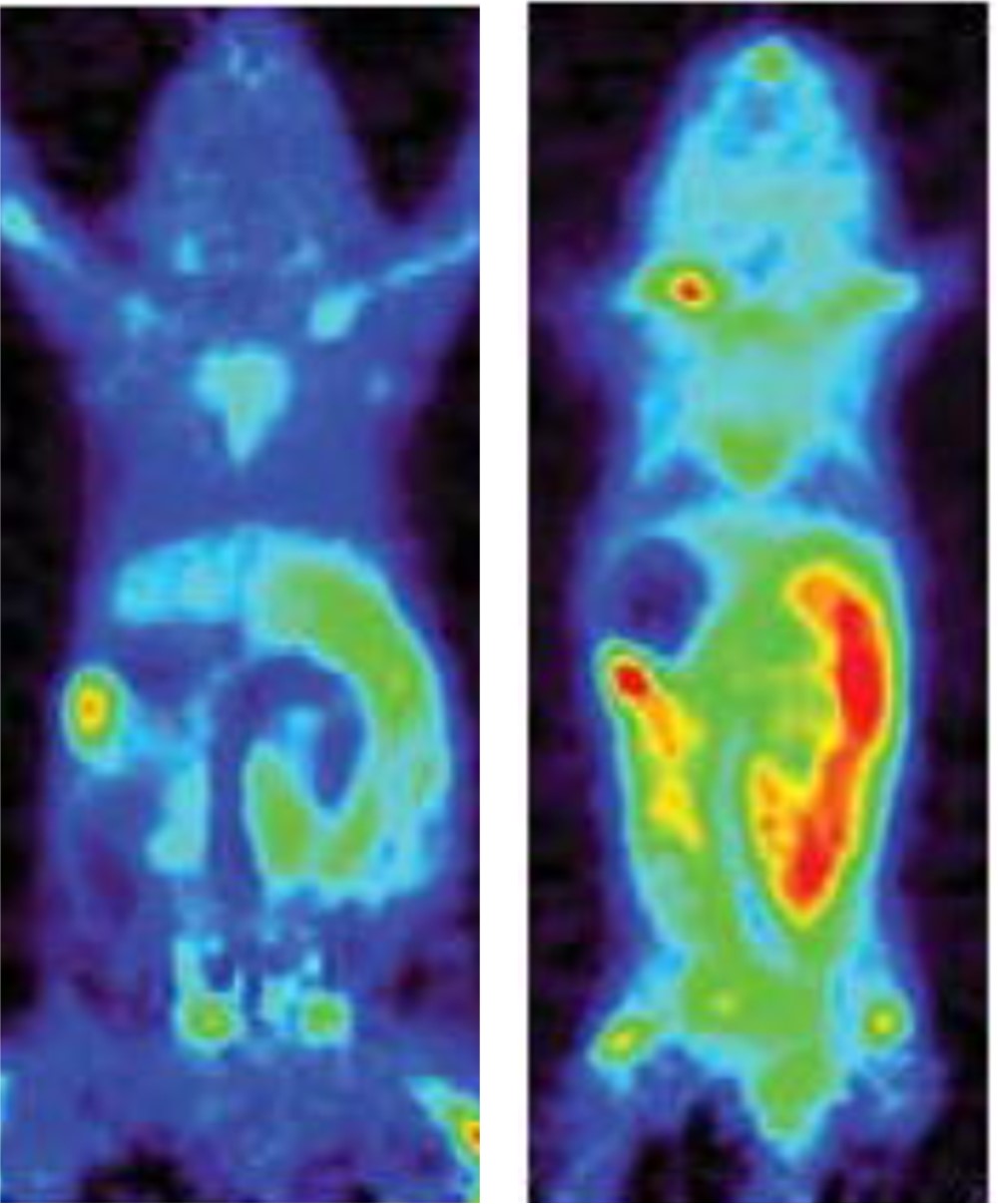
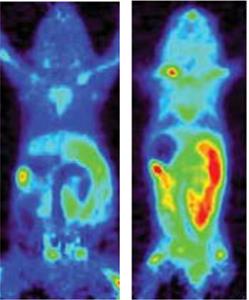
Figure 1. PET scan images showing presence of target enzyme dCK in a normal mouse (left), and one with Crohn’s disease (right).
Sources: NEnglJMed.2020 Dec 31;383(27); Lancet.2017 Apr 29;389; Gastro.2010 Apr138; AmJGastro.2009 Mar;104.
Note: Statements taken from the official NIH peer review written summary reflect their personal scientific assessment and are not official NIH endorsements.
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. The FDA has designated TRE-515 a Fast Track drug for prostate cancer and an Orphan Drug for two autoimmune neurologic diseases. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are "forward-looking statements," which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/a36a116d-4f72-40ab-bf03-916d2732d57b

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.